Training on GCP and Regulatory & Ethical requirements for Clinical Trials
The Clinical Development Services Agency (CDSA), THSTI organized a two-day training program on Good Clinical Practice and current regulatory and ethical requirements for conducting clinical trials and research in India. The program took place from July 29-30, 2024. The focus of the program was to enhance the capabilities of researchers to improve research outputs and outcomes. To develop the capacity and capability of research staff and M.Sc. students (2022-2024) to participate in and lead clinical research and trials for the public good, 70 participants from THSTI attended the program. The program included hands-on activities, quizzes, and interactive sessions. The training concluded with an exit assessment leading to the certification of the participants.



 02 Feb 2026
02 Feb 2026
 27 Jan 2026
27 Jan 2026
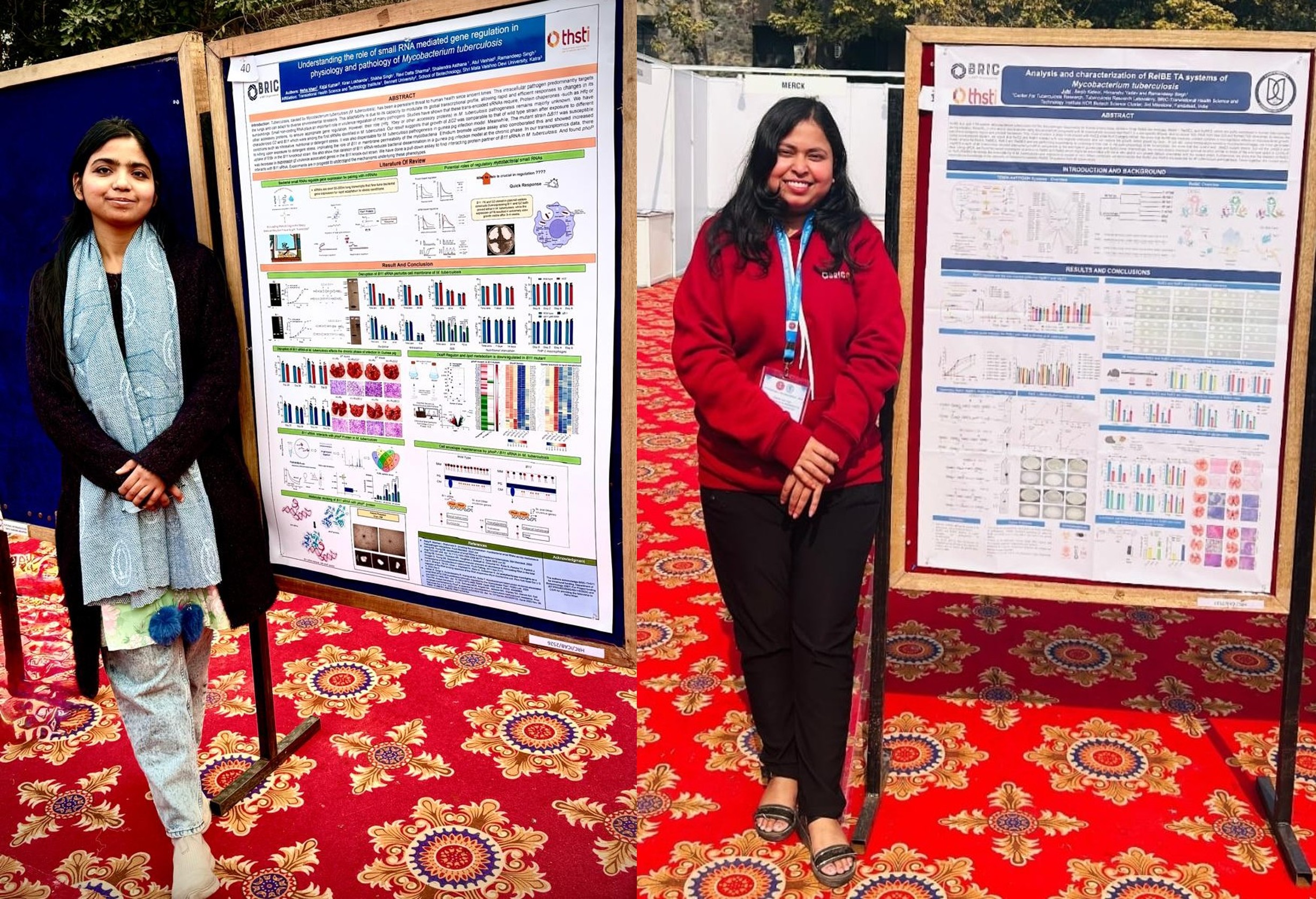 27 Jan 2026
27 Jan 2026
 22 Jan 2026
22 Jan 2026
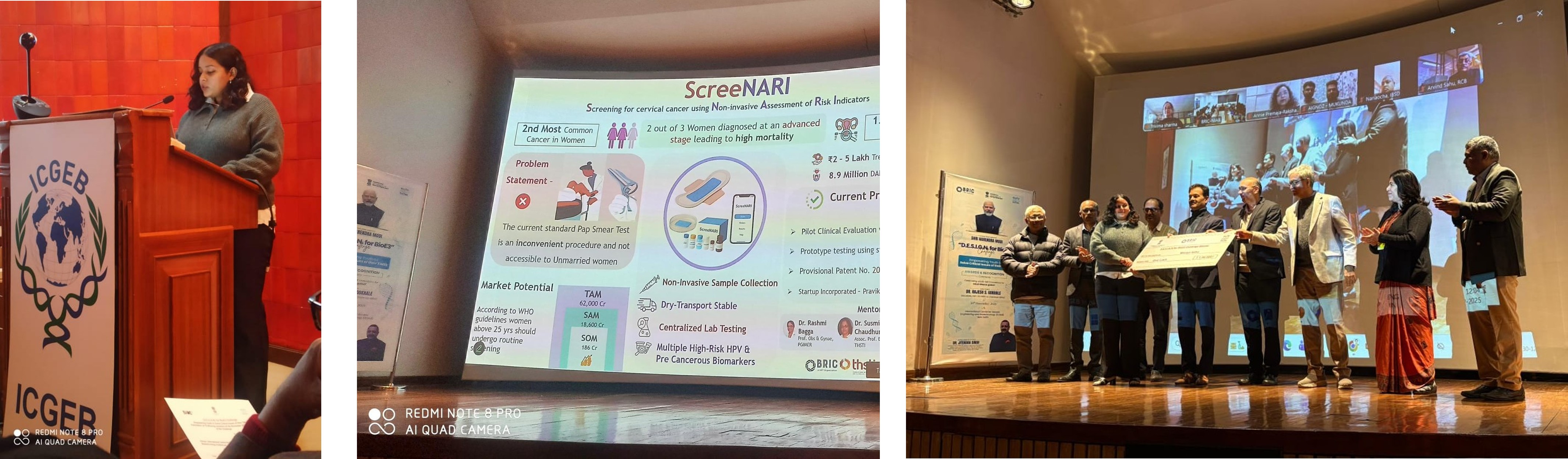 30 Dec 2025
30 Dec 2025