Mission
ERID’s mission is to support and facilitate innovation in the translational research setting at THSTI in a one-stop service platform and enhance the organization’s profile to the rest of the world.
Goals/ Objectives
- To create a conducive system of innovation management.
- To create a transparent machinery of proper implementation of biosafety, environmental safety, animal & human ethics.
- To create a support system for extramural fund generation and build effective investor relations.
- To create an effective communication to enhance the profile of the organization.
ERID Structure

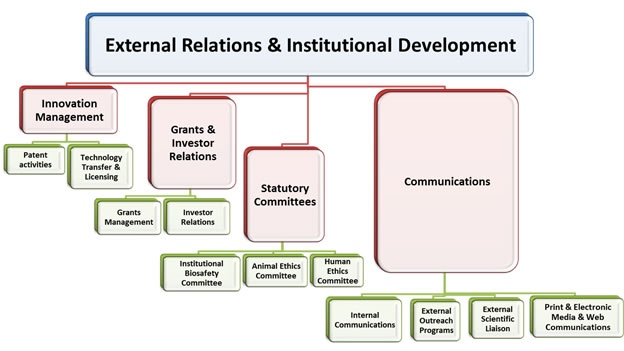
Significance of ERID at THSTI
- ERID will provide support throughout the lifecycle of a THSTI research project. Its functions include supporting PIs in finding funding, receiving biosafety approval, human / animal ethics approval, negotiating and approving research contracts, meeting grant requirements, and disseminating the scientific results
- ERID will bring together R&D facilitation and communications functions under one umbrella as a “one-stop service point,” to ensure quality research, policy compliances, increased administrative efficiency, timely completion of study reports and effective technology transfer or licensing, and also effective communication to create a significant impact to the outside world.
Resources
- Format for submission to IBSC [PDF]
- Format for submission to IEC (HR) [PDF]
- Form B for submission to AEC [PDF]
- Insitutional Biosafety Manual [PDF]
- Research misconduct statement-DBT [PDF]
- Approved Guidelines for AEC [PDF]
- National Ethical Guidelines for Biomedical and Health Research Involving Human Participants (2017) [PDF]
- National Ethical Guidelines for Biomedical Research Involving Children (2017) [PDF]
