CDSA and THSTI organize a meeting to discuss Common Review Process for multicentre studies in India
CDSA and THSTI organized this meeting in collaboration with the ICMR on 6th August 2018 at THSTI, Faridabad. It was attended by senior clinical researchers, trialists, members of ethics committees, independent ethics experts from different institutions in the country and a representative from CDSCO.
A common review process for ethical approval of multicentre studies and a pilot undertaken using the process was presented. The discussions focused on how the proposed common review process would apply to multicentre clinical trials. There was much debate on roles and responsibilities of the ethics committee chosen to undertake the common review (designated ethics committee) and of the ethics committees of the participating sites. The general consensus was in support of a common review process as it was felt that it would enhance the quality of the ethical review.
The final recommendations will be added as an appendix to the recently released ICMR National Ethical guidelines for Biomedical and Health research involving human participants released in 2017.


 22 Jan 2026
22 Jan 2026
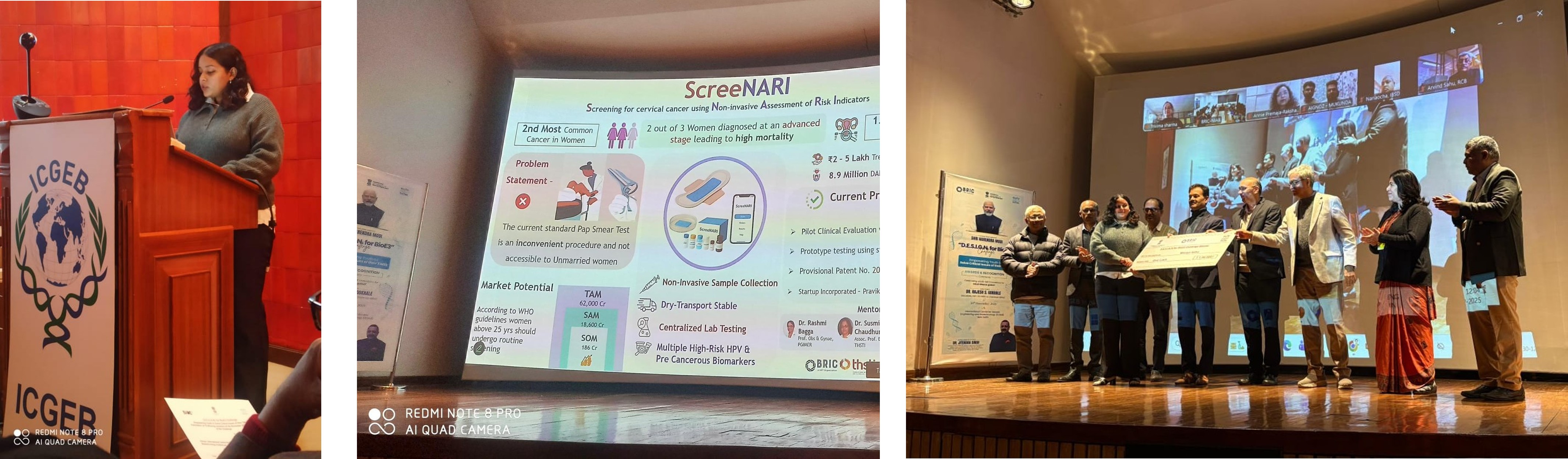 30 Dec 2025
30 Dec 2025
 29 Dec 2025
29 Dec 2025
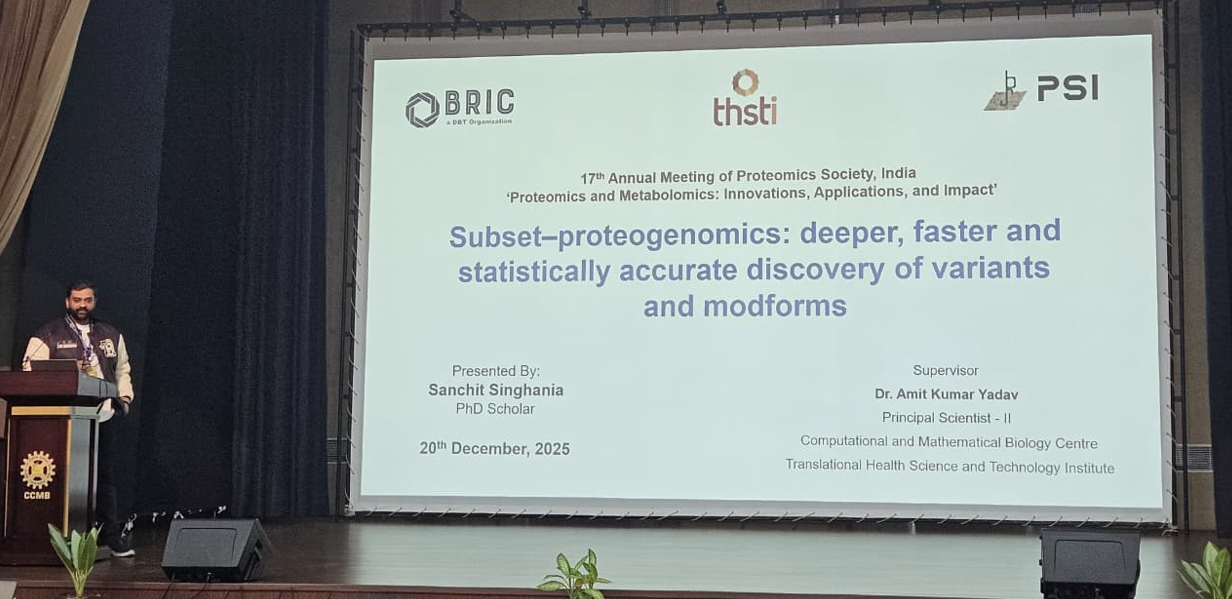 29 Dec 2025
29 Dec 2025
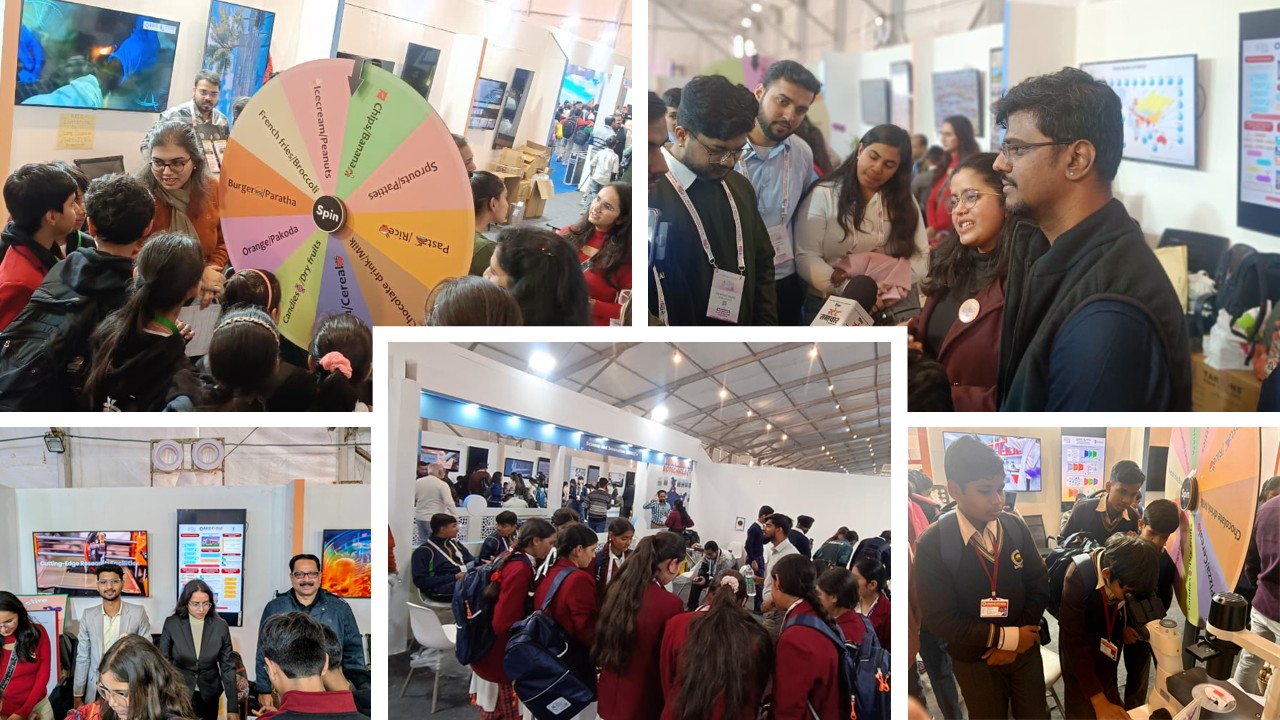 10 Dec 2025
10 Dec 2025