CDSA and C-CAMP co-host National workshop on regulatory compliance for accelerating innovations
The Department of Biotechnology (DBT) and Central Drugs Standard Control Organisation (CDSCO) along with Biotechnology Industry Research Assistance Council (BIRAC) and CDSA had organized a one-day interactive program, “National workshop on regulatory compliance for accelerating innovations” on April 09, 2019 with Centre for Cellular And Molecular Platforms (C-CAMP), Bangalore. This was one of the six national programs planned across the nation.
The workshop was attended by senior representatives from Government organisations, academia, medical device industry, in vitro diagnostics, new drugs, biopharma, phytopharma representing various start-ups, hospitals, institutes etc. There were total 86 participants representing 80 institutions who attended the workshop. There were 17 faculties including senior experts, present and former regulators from CDSCO; BIS (Bureau of Indian Standards); CDSA, THSTI (Translational Health Science & Technology Institute); NABCB (National Accreditation Board for Certification Bodies), QCI (Quality Council of India) and NIB (National Institute of Biologicals). 12 CDSCO senior officers attended this program, which included Drugs Controller, Government of Karnataka; 2 Deputy Drugs Controller and 2 Assistant Drugs Controller. There were three major themes: medical devices, new drugs & phyto-pharma, and biopharma. The regulators addressed all questions asked by the participants. Biopharma was attended by 21, new drug and phytopharmaceuticals by 26 and medical devices and in vitro diagnostics by 39 participants respectively.





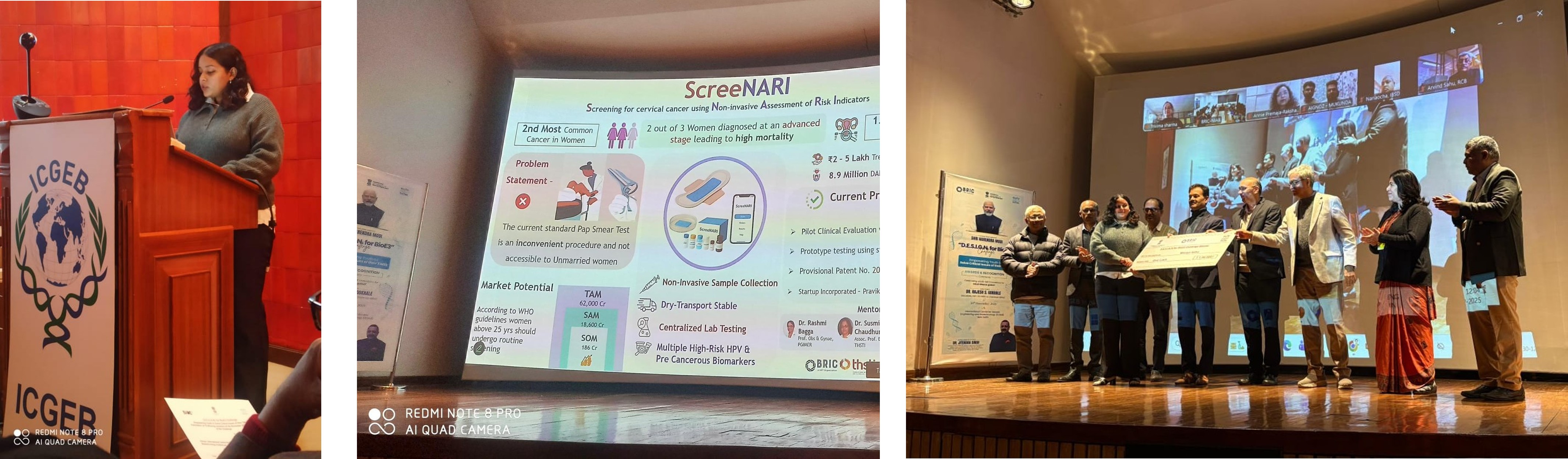 30 Dec 2025
30 Dec 2025
 29 Dec 2025
29 Dec 2025
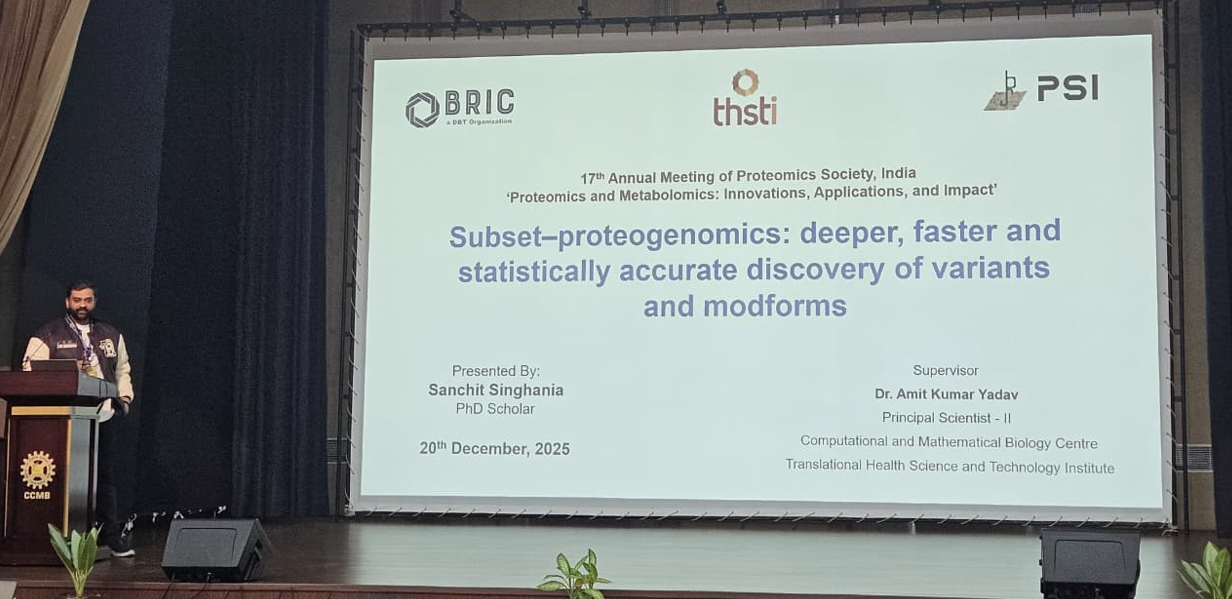 29 Dec 2025
29 Dec 2025
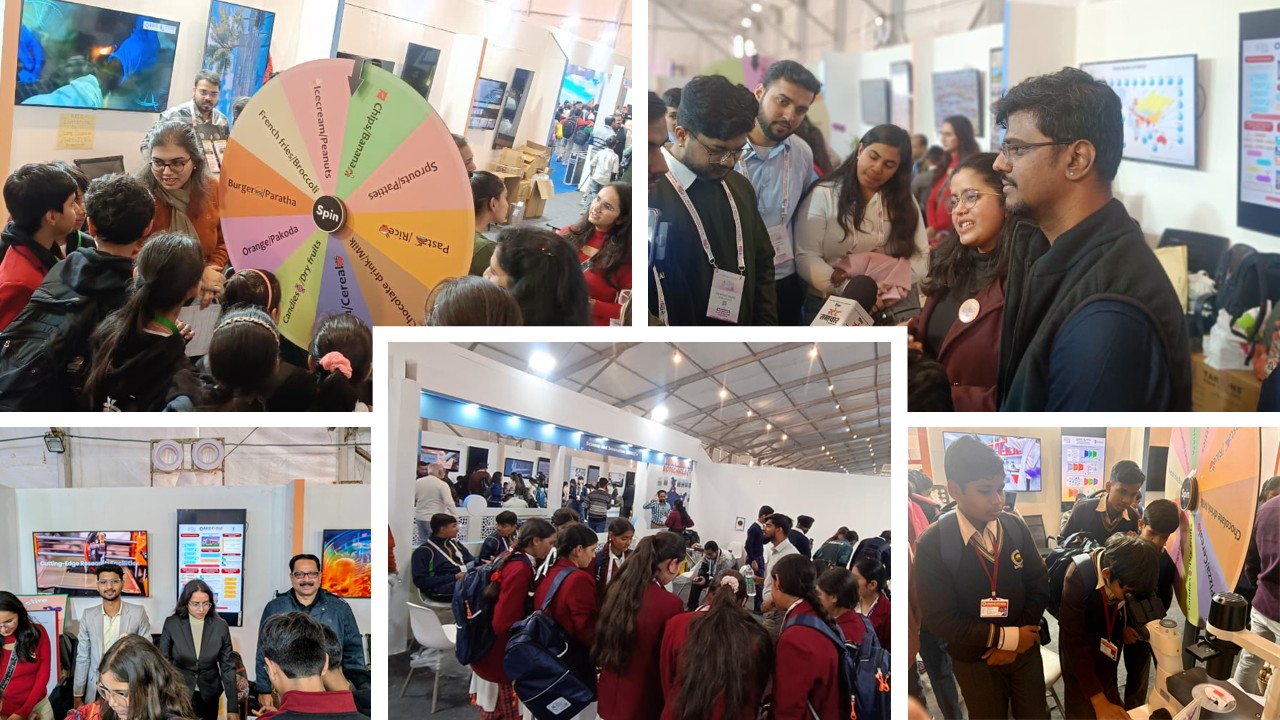 10 Dec 2025
10 Dec 2025
 10 Dec 2025
10 Dec 2025